ELISpot and FluoroSpot Assay Market Size to Gain USD 576.59 Million by 2034
ELISpot and FluoroSpot Assay Market Size and Forecast 2025 to 2034
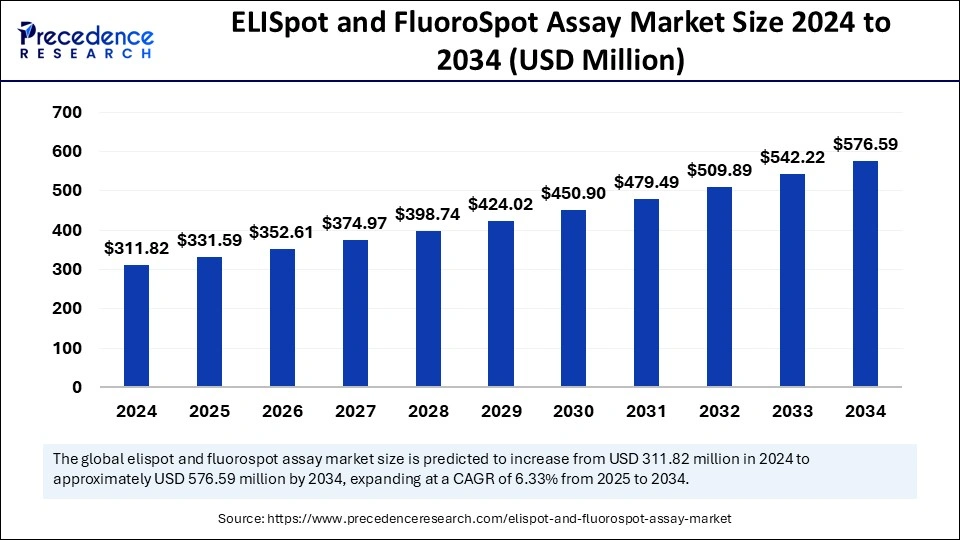
The global ELISpot and FluoroSpot assay market size surpassed USD 311.82 million in 2024 and is projected to gain around USD 576.59 million by 2034 with a CAGR of 6.33%.
ELISpot and FluoroSpot Assay Market Key Points
-
In 2024, North America held the top position globally with a market share of 35%.
-
Asia Pacific is poised to achieve the highest CAGR of 7.24% during the forecast period.
-
Europe is likely to record substantial gains in market share in the coming years.
-
The assay kits segment was the market leader by product in 2024, securing a 50% share.
-
The analyzers product category is expected to exhibit rapid growth between 2025 and 2034.
-
Diagnostic applications led the market in 2024, comprising 67% of the total share.
-
Research applications are projected to expand at the fastest CAGR over the next several years.
-
Hospitals and clinical labs remained the top end-user segment in 2024 with a 47% contribution.
-
Biopharmaceutical companies are likely to see the most rapid growth among end-use sectors through the forecast period.
The Transformative Role of AI in the ELISpot and FluoroSpot Assay Market
Artificial Intelligence (AI) is playing a transformative role in the ELISpot and FluoroSpot Assay Market by enhancing accuracy, speed, and scalability in immune monitoring and cell-based assays. AI-powered image analysis tools enable precise detection and quantification of cytokine-secreting cells by distinguishing true signals from background noise.
This significantly reduces manual errors and increases the reproducibility of results, especially in high-throughput environments such as clinical diagnostics, vaccine development, and immunotherapy research.
Beyond image analysis, AI also streamlines workflow automation and data interpretation. By integrating assay results with multi-omics data and predictive models, AI facilitates deeper insights into immune responses and helps identify disease-specific biomarkers.
Additionally, cloud-based AI platforms support remote analysis and centralized data management, making collaboration across research sites more efficient. As a result, AI integration is emerging as a key driver of innovation and competitiveness in the ELISpot and FluoroSpot market.
What is ELISpot?
ELISpot (Enzyme-Linked ImmunoSpot) is a highly sensitive, cell-based assay used to detect and quantify individual immune cells secreting specific cytokines or other proteins. It is primarily used to monitor immune responses, especially T-cell activity, by measuring how many cells in a sample produce a particular cytokine such as IFN-γ or IL-2.
Key Features:
-
Detects cytokine secretion at the single-cell level
-
Provides quantitative and functional data on immune cells
-
Widely used in vaccine development, infectious disease research, autoimmune studies, and cancer immunology
What is FluoroSpot?
FluoroSpot is an advanced version of ELISpot that uses fluorescent detection instead of enzymatic color change. This allows simultaneous detection of multiple cytokines from a single cell, enabling multiplexing in one assay.
Key Features:
-
Detects polyfunctional cells secreting multiple cytokines
-
Uses fluorescent antibodies to identify different cytokines in one well
-
Ideal for complex immune profiling, especially in studies involving T-cell polyfunctionality
Applications of Both Assays:
-
Vaccine efficacy studies (such as COVID-19, HIV, and tuberculosis)
-
Immunotherapy research in cancer and autoimmune diseases
-
Drug development and clinical trials
-
Transplantation research to monitor immune rejection or tolerance
Market Overview
The ELISpot and FluoroSpot Assay market is evolving with rising demand in both academic and commercial research sectors. These assays are valued for their ability to deliver detailed insights into immune function, especially in applications involving T-cell monitoring, which is critical for vaccine development and cancer immunotherapy.
ELISpot and FluoroSpot Assay Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 576.59 Million |
| Market Size in 2025 | USD 331.59 Million |
| Market Size in 2024 | USD 311.82 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 6.33% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product , Application, End Use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
ELISpot and FluoroSpot Assay Market Dynamics
Market Drivers
Rapid growth in cell-based research, expansion of personalized medicine, and increasing clinical trial volume are key market drivers. The demand for accurate immune monitoring tools is being reinforced by developments in cancer immunotherapies and infectious disease surveillance.
Opportunities
Opportunities exist in the expansion of diagnostic applications, especially with multiplex FluoroSpot assays offering simultaneous detection of multiple cytokines. The adoption of cloud-based platforms and digital imaging systems also creates prospects for improved assay accessibility and data management.
Challenges
The market faces obstacles including the technical difficulty of assay execution and data analysis, particularly for less experienced labs. Regulatory hurdles and the cost of adopting new technology can also slow down market penetration, especially in developing countries.
Regional Insights
North America continues to dominate due to technological advancements and a strong base of research institutions. Asia Pacific shows promising growth, backed by increasing investments in biotechnology and rising awareness of advanced diagnostic methods. Europe remains a key contributor with its emphasis on precision medicine and academic research funding.
ELISpot and FluroSpot Assay Market Comapnies

- Abcam Limited
- Bio-Techne Corporation0
- BD
- Cellular Technology Limited
- Mabtech
- Autoimmun Diagnostika GmbH
- Lophius Biosciences GmbH
- Bio-Connect B.V.
- Oxford Immunotec
- U-CyTech
Leader’s Announcements
- In March 2025, Beckman Coulter Diagnostics, announced the U.S. FDA 510 (k) clearance for their novel DxC 500i Clinical Analyzer which is an integrated clinical chemistry and immunoassay analyzer. Kathleen Orland, Chief Portfolio Officer for Beckman Coulter Diagnostics, said that, “Innovations like the DxC 500i Clinical Analyzer enable Beckman Coulter to address the needs of networked laboratories with specific solutions for satellite or independent laboratories, as well as core laboratories. Beyond ensuring appropriate throughput levels for a networked lab, Beckman Coulter’s common reagents and consumables across its scalable clinical chemistry and immunoassay portfolio enables common reference ranges, offering IDNs strategic benefits in patient care and inventory management.”
- In January 2025, CellFE, a disruptive microfluidics company leading in non-viral gene editing technology announced the launch of its CellFE T-Rest (Resting T Cell Kit) which is a cell manufacturing transfection media product designed specifically for resting (quiescent) T cell workflows. Alla Zamarayeva, CEO of CellFE, said that, “We’re thrilled to launch T-Rest – the first-in-class resting T cell commercial product – to offer a new paradigm for cell therapy manufacturing. By enabling efficient editing of resting T cells, we address the durability and safety concerns that currently challenge cell therapy manufacturers.”
ELISpot and FluoroSpot Assay Market Recent Developments
- In October 2024, BioVaxys Technology Corp., a clinical stage biopharmaceutical company, declared that DPX, an innovative immune educating delivery platform developed by the company assists in recruiting and activating unique subsets of antigen presenting cells (APCs) for accelerating the immunogenicity of antigens further showing enhanced immune activation in comparison to aqueous and emulsion-based antigen delivery systems.
- In May 2024, Revvity Inc., a life sciences and diagnostics company, launched the Auto-Pure 2400 liquid handler from Allsheng which can be utilized with the T-SPOT.TB test for detecting latent tuberculosis infection (LTB1). The test is an interferon-gamma release assay (IGRA) predicated on the enzyme-linked immunospot (ELISPOT) technology and the Auto-Pure 2400 automated liquid handling platform is laced with integrated magnetic cell isolation technology.
Segments Covered in the Report
By Product
- Assay Kits
- Analyzers
- Ancillary Products
By Application
- Research Applications
- Diagnostics Applications
By End-use
- Hospital and Clinical Labs
- Academic and Research Institutes
- Biopharmaceutical Companies
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Also Read: Surgical Fluid Waste Management System Market
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/
