Blood Pressure Monitoring Devices Market Size to Worth USD 4.35 Bn by 2034
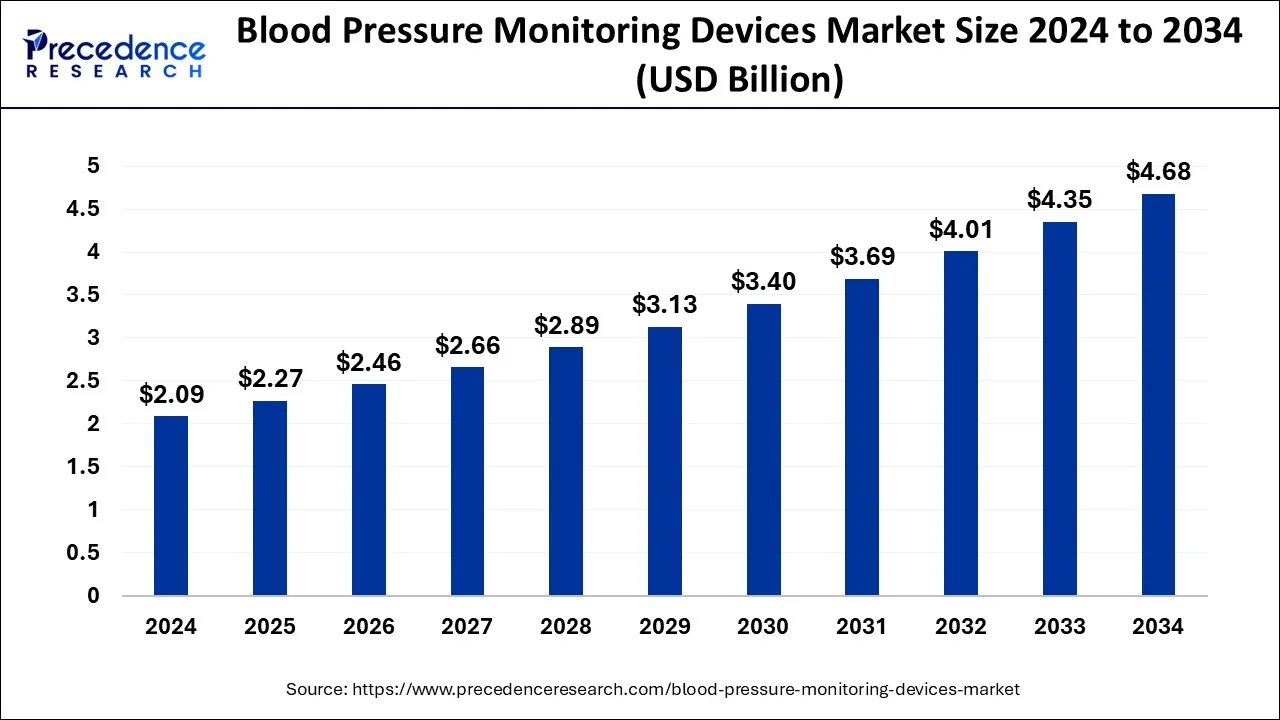
The blood pressure monitoring devices market size is expected to worth around USD 4.35 billion by 2034 from USD 2.09 billion in 2024 with a notable CAGR of 8.49%.
Get Sample Copy of Report@ https://www.precedenceresearch.com/sample/1090
Table of Contents
ToggleKey Points
- In 2024, North America maintained the highest market share globally, reaching 37.44%.
- The digital blood pressure monitor segment secured the top position by product, with a 35.80% market share.
- Hospitals dominated the end-user category, accounting for 59.34% of the total revenue share.
Market Dynamics
Blood Pressure Monitoring Devices Market Drivers
The growing burden of hypertension and lifestyle-related diseases has significantly increased the demand for blood pressure monitoring devices. With a rising geriatric population and growing awareness about the risks of high blood pressure, more individuals are opting for regular monitoring.
The shift towards home healthcare solutions and self-monitoring devices has further boosted market demand. Additionally, technological advancements in digital monitoring systems, including AI-powered diagnostics and cloud-based health tracking, are transforming the industry.
Blood Pressure Monitoring Devices Market Opportunities
The market presents significant growth opportunities with the increasing integration of wearable technology and mobile health applications. Companies are focusing on developing smart blood pressure monitors that sync with smartphones, allowing users to track their health seamlessly.
Government initiatives aimed at improving healthcare accessibility and affordability in developing countries also create a positive market outlook. Additionally, the expansion of online retail channels has made it easier for consumers to purchase and access high-quality monitoring devices.
Blood Pressure Monitoring Devices Market Challenges
Challenges such as the high cost of advanced monitoring devices and the lack of affordability in low-income regions hinder market growth. Many consumers still rely on traditional monitoring methods due to concerns over the accuracy and reliability of digital blood pressure monitors.
Additionally, regulatory compliance and certification processes for medical devices are stringent, which slows down product approvals. The presence of counterfeit products in the market also poses a challenge, affecting both consumer trust and market reputation.
Blood Pressure Monitoring Devices Market Regional Analysis
North America holds the largest share in the market due to a strong healthcare system, high awareness levels, and advanced technological adoption. Europe follows closely, supported by government-led healthcare initiatives and a growing elderly population.
The Asia Pacific region is emerging as a high-growth market, with increasing investments in healthcare infrastructure and a rising focus on non-communicable disease prevention. Meanwhile, Latin America and the Middle East & Africa are experiencing gradual market growth, driven by improved healthcare access and growing awareness of hypertension management.
Blood Pressure Monitoring Devices Market Companies
- Koninklijke Philips N.V.
- General Electric Company
- A & D Company, Limited
- SunTech Medical, Inc.
- Welch Allyn
- American Diagnostic Corporation
- Briggs Healthcare
- Withings
- Spacelabs Healthcare
- GF HEALTH PRODUCTS, INC.
- Kaz, A Helen of Troy Company
- Rossmax International Limited
- Microlife Corporation
Recent Developments
- In December 2024, Withings’ BPM Pro 2, a cellular blood pressure monitor designed to enhance remote patient care for those with heart failure, has received approval from the U.S. Food and Drug Administration (FDA). The gadget was first introduced in October and went on sale in the United States on January 1 2025.
- In November 2024, in a move that marks the company’s first market expansion outside of Europe, Aktiia’s continuous blood pressure monitoring (CBPM) wristband was approved by Health Canada and introduced there
Segments Covered in the Report
By Product
- Digital Blood Pressure Monitor
- Wrist
- Arm
- Finger
- Sphygmomanometer
- Ambulatory Blood Pressure Monitor
- Instruments & Accessories
- Blood pressure cuffs
- Reusable
- Disposable
- Others
- Blood pressure cuffs
- Transducers
- Reusable
- Disposable
By End-User
- Ambulatory Surgical Centers & Clinics
- Hospitals
- Home Healthcare
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Malaysia
- Philippines
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of the Middle East & Africa
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/
